Babak Azizzadeh, M.D., F.A.C.S.
Facial Paralysis Institute
Attending Surgeon – Facial Plastic & Reconstructive Surgery
Cedars-Sinai Medical Center
Assistant Clinical Professor of Surgery
David Geffen School of Medicine at UCLA
Intro to Partial Facial Paralysis
Facial paralysis is a condition that can affect some or all of the branches of the facial nerve. Facial nerve disorders can lead to poor facial aesthetics and functional deficits that can severely affect patient quality of life. Furthermore, in many patients with partial facial paralysis, synkinesis of the facial musculature will complicate the treatment goals.
The goals of facial paralysis reconstruction include achieving symmetry at rest, dynamic facial movement, and appropriate eyelid function. This chapter aims to address the non-surgical and surgical methods of lower facial reanimation surgery with a focus on delineating static and dynamic options.
History
The possibility of facial nerve surgery was initially conceived by Sir Charles Bell in 1821 when he established that the facial nerve innervates the muscles of facial expression.1 Historically, facial nerve paralysis was treated medically with topical ointment, medicine, and electrotherapy.2 The first nerve transfer was performed by Drobnick in 1879 when he anastomosed the spinal accessory nerve to a dysfunctional facial nerve.3 Manasse and Korte, in the early 1900s, performed hypoglossal to facial nerve substitution in addition to the spinal accessory nerve.4,5 In that same time period, Stacke resected a portion of the facial nerve and juxtaposed the cut ends.6
The first facial nerve graft within the temporal bone was performed by Bunnell in 1927.7 In addition to Bunnell’s work, Lathrop and Myers demonstrated that the facial nerve could regenerate and that facial movement could be improved with facial nerve reconstruction. 8,9 Sir Charles Balance, the founder and first president of the Society of British Neurological Surgeons, showed that nerve grafts for the facial nerve had more favorable results than those obtained by anastomosis of the facial nerve to the hypoglossal and glossopharyngeal nerves. 10,11 Lexer in 1908 described temporalis and masseter regional muscle transposition techniques.12 These dynamic techniques were also later reported by Erlacher in 1915 and Owens in 1947.13
The modern era of free tissue transfer introduced novel treatment modalities that have subsequently revolutionized facial reanimation surgery recovery. Scaramella described cross face nerve grafts in 1970. 14,15,16 Thompson proposed free muscle transplantation to reanimate the paralyzed face that was subsequently supported by Rubin,Harii, Ohmore, and Torii.17,18,19 Cross facial nerve grafts followed by vascularized muscle free flaps paved the way for modern facial reanimation surgery.20
Facial Nerve Anatomy And Function
The facial nerve is comprised of branchial motor, parasympathetic, sensory and taste components. The special visceral efferent fibers also supply the stapedius, stylohyoid, posterior digastric and buccinators muscles. The parasympathetic or general visceral efferent fibers innervate the lacrimal, submandibular and sublingual glands. The sensory or general sensory afferent fibers supply sensation to the auricular concha, postauricular skin and external auditory canal, as well as part of the tympanic membrane. The special visceral afferent fibers are responsible for taste mechanism of the anterior 2/3 of the tongue and palate.
The branchial motor and visceral efferent fibers begin in the premotor cortex then course through the corticobulbar tract to the bilateral facial nuclei in the pons to innervate the muscles of facial expression. Fibers innervating the forehead receive bilateral innervation from the upper motor neurons while the lower face receives contralateral fibers only from the upper motor neurons. The extracranial portion of the facial nerve begins as the nerve emerges from the stylomastoid foramen. The nerve divides into two major divisions at the pes anserinus into an upper and lower division and passes through the parotid gland. Within the parotid gland, the nerve further divides into five major branches: temporal, zygomatic, buccal, marginal mandibular and cervical. Table 1 outlines the various facial expressions controlled by the motor branch of the facial nerve.
Table 1. Facial Nerve Branch Function
| Temporal/Frontal | Elevates the eyebrows Pulls eyebrow downward and medially Pulls medial eyebrow downward |
| Zygomatic | Closes eyelids Contributes to buccal branch in elevating oral commissure |
| Buccal | Flares nostrils Elevates upper lip and mid-nasolabial fold Elevates medial nasolabial fold and nasal ala Compresses nostrils |
| Marginal Mandibular | Pulls down corner of mouth Pulls down lower lip Pulls chin upward |
| Cervical | Pulls down corner of mouth |
Facial paralysis can result from a variety of factors, including congenital, iatrogenic, idiopathic, infectious, metabolic, neoplastic, neurologic, toxic and trauma factors (Table 4). Paralysis of the facial musculature results in aesthetic, functional and psychosocial morbidity.4 Unilateral cases cause facial asymmetry at rest and/or during animation. However, individuals with bilateral facial paralysis can have a more symmetrical appearance, and the psychological effect of not being able to effectively express one’s emotions can be significant.
Facial paralysis sometimes leads to aesthetic deformity and psychosocial issues, and the functional consequences can lead to even more significant morbidity and deteriorating quality of life.4 Patients with facial palsy often do not communicate effectively, experience oral incompetence and may have significant tension from synkinesis. Lagophthalmos and lower lid malposition can lead to corneal desiccation, ulceration and eventual blindness.
Patient Evaluation
Chapter XX will discuss the details of facial paralysis evaluation and should serve as an excellent reference. In terms of individuals seeking lower facial reanimation, there are a few key issues that need to be determined. Most patients have likely been examined by a clinician and given a diagnosis for the etiology of their facial paralysis. While the physical manifestations of facial nerve paralysis are distressing for all patients, it is important to take a detailed history and perform a thorough physical examination to determine the underlying etiology. It is extremely important not to be sidetracked by an established diagnosis at the time of evaluation. A significant amount of time needs to be spent on confirming the correct diagnosis in this patient population. Many patients diagnosed with Bell’s palsy may harbor serious malignancies.
Bell’s palsy is one of the primary causes of facial paralysis in the United States, and it is not identical to facial paralysis. Whereas facial paralysis occurs due to salivary gland inflammation, tumors or other causes, the root cause of Bell’s palsy is believed to be the reactivation of herpes simplex virus (HSV).
When Bell’s palsy occurs, HSV is reactivated in the temporal bone, which is located behind the ear. This causes the facial nerve to swell and stop working and immediately hinders an individual’s ability to smile and make other facial expressions.
In addition to the identification and diagnosis of Bell’s palsy or facial paralysis, other key issues need to be clarified during a patient evaluation, and these include: severity of facial paralysis and synkinesis, duration of Bell’s palsy, patient age, functional deficits and long-term dynamic facial reanimation goals. Individuals with partial paralysis who have synkinesis will often require a different treatment protocol than those with complete flaccid paralysis. Duration of facial palsy is also critical, as most surgical interventions are typically performed only after the regenerative functions of the nerve have been clearly established (typically one year). The etiology of the paralysis will help guide the surgeon as what is the appropriate time frame to wait before initiating treatment. Finally, the patient’s long-term goals need to be clearly understood. The main complaint from patients with facial paralysis is oral incompetence, facial asymmetry and inability to generate a smile. Some individuals just desire to improve symmetry at rest and reduce functional deficits while others desire spontaneous dynamic facial movements.
During the physical examination, the eyes are inspected for narrowing or widening of the aperture, degree of lagophthalmos, Bell’s palsy phenomenon, lower eyelid position and laxity (snap test). The facial movements corresponding to each of the facial nerve branches must be evaluated carefully by asking the patient to raise their eyebrows, close their eyes, wrinkle their nose, smile, show their teeth and pucker their lips. The key factor is to determine whether the patient has complete flaccid paralysis or partial paralysis with muscle tone. Patients with partial paralysis need to be further inspected for any evidence of synkinesis, which typically involves simultaneous and uncoordinated oculofacial muscle contractions (such as orbicularis oculi contraction while trying to smile). Synkinesis often leads to “auto-paralysis” where simultaneous activation of the orbicularis oris, buccinators, oral commissure elevators and depressors result in a frozen smile and prevent the patient from having a true smile mechanism.
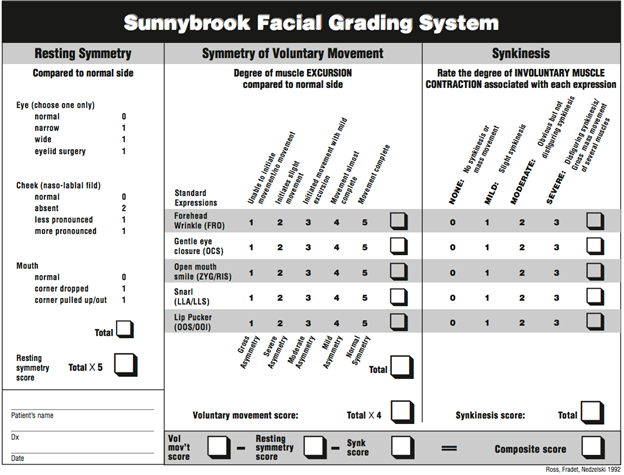
The House-Brackmann and Sunnybrook Facial Grading System (SFGS) are useful tools to evaluate facial palsy and synkinesis (Tables 2 and 3). The House-Brackmann grading system is a six-point scale, while the SFGS evaluates synkinesis at rest, during movement and with voluntary movement.6 The scale is continuous from 0-100, where 0 indicates complete paralysis and 100 is normal.10
In clinical practice, the senior author categorizes facial palsy patients into 5 categories:
Type A– Normal facial function
Type B– Partial paralysis with mild synkinesis
Type C– Partial paralysis with moderate to severe synkinesis
Type D– Partial paralysis without synkinesis
Type E– Complete facial paralysis
Table 2. House Brackmann Grading System10
| Grade | Definition |
| I. Normal | Normal symmetric facial function in all areas |
| II. Mild Dysfunction | Slight weakness noticeable on close inspection Complete eye closure with minimal effort Slight asymmetry of smile with maximal effort Synkinesis barely noticeable, contracture, or spasm absent |
| III. Moderate Dysfunction | Obvious but not disfiguring weakness May not be able to lift eyebrow Complete eye closure and strong but asymmetrical mouth movement with maximal effort Obvious but not disfiguring synkinesis, mass movement, or spasm |
| IV. Moderately Severe Dysfunction | Obvious disfiguring weakness Inability to lift brow Incomplete eye closure and asymmetry of mouth with maximal effort Severe synkinesis, mass movement, spasm |
| V. Severe Dysfunction | Motion Barely perceptible Incomplete eye closure Slight movement in the corner of the mouth Synkinesis, contracture, and spasm usually absent |
| VI. Total Paralysis | No movement, loss of tone, synkinesis, contracture, or spasm |
Table 3. Sunnybrook Facial Grading System10
Reprinted with permission from Chee, Gerard H.; Nedzelski, Julian M.:
Facial Nerve Grading Systems; Facial plast Surg 2000; 16: 315-324. Thieme.
To complete the physical exam, the remaining cranial nerves should be evaluated and radiographic imaging (CT or MRI) may be required. Electromyographic studies can be useful in determining the viability of the muscle fibers; however, in patients with long-standing facial paralysis, a physical examination will provide enough information about the muscle tone and function. Preoperative photography and video assessment at rest and during animation is recommended. A multidisciplinary approach with a physical therapist that is knowledgeable and skilled in working with facial paralysis patients is strongly recommended for optimizing outcomes.
Etiology
Prior to any intervention, appropriate evaluation of the patient in an effort to determine the underlying etiology is critical. Facial paralysis can be congenital or acquired (Table 4).
The most common etiology of unilateral facial paralysis is idiopathic, also referred to as Bell’s palsy.11 Approximately 85% of patients with Bell’s palsy start to have spontaneous recovery within a few weeks after onset. The remaining 15% may not experience facial movement for up to six months; however, most of these patients will have some level of recovery. The duration of time is directly correlated to higher incidence of synkinesis, loss of mimetic function and contracture. The likelihood of recovery is best for younger age groups.7
The recovery period for patients dealing with Bell’s palsy or facial paralysis varies. An individual requires close evaluation throughout the course of a treatment to ensure his or her recovery stays on track. That way, this individual can receive comprehensive support and enjoy the full results of dynamic facial reanimation.
Table 4. Etiology of Facial Nerve Paralysis
Congenital (e.g. Moebius Syndrome, Craniofacial microsomia)
Trauma (e.g. temporal bone fracture, laceration)
Tumor (e.g. cerebellopontine angle tumor, facial neuroma, malignant head and neck neoplasm)
Iatrogenic (e.g. acoustic neuroma resection, parotidectomy, temporal bone resection, neck dissection, rhytidectomy)
Infectious (e.g. Lyme Disease, Ramsay Hunt)
Melkerson-Rosenthal
Idiopathic (Bell’s palsy)
If facial paralysis is caused by a traumatic event and the nerve ends can be identified and stimulated, repair within the first three days of the injury yields the highest likelihood of recovery.21 Generally, if damage is to the buccal or zygomaticus branches medial to the lateral canthus, the nerve is able to recover on its own and no repair is indicated. Proximal injuries to these branches will require surgical repair. The buccal and zygomatic branches have extensive arborization and therefore the likelihood of permanent paralysis is less common than trauma to frontal and marginal mandibular branches, which are terminal branches. In acoustic neuroma resection and parotid neoplasms, the facial nerve may be intentionally sacrificed for tumor resection. In temporal bone fractures, the facial nerve may be transected, crushed or impinged leading to facial palsy.
Non-Surgical Intervention
The main non-surgical treatment options for lower facial reanimation are the use of neuromodulators, injectable fillers and neuromuscular rehabilitation. Other chapters in this text will discuss these options in further detail; however, the authors would like to emphasize that neuromodulators such as botulinum toxin-A (BTX-A) play a crucial role in creating facial symmetry, improving synkinesis, increasing oral commissure excursion and reducing functional deficits.8,22 Patients with Type B and C partial paralysis with synkinesis and congenital unilateral lower lip palsy (CULLP) are typically the best candidates. Synkinetic patients typically have simultaneous activation of oral commissure elevators, orbicularis oris, buccinator and lower lip depressor activity. They therefore can have improvement in their dynamic smile function by reducing the downward force of depressors such as the depressor labii inferioris, risorius and depressor anguli oris.
Botox is among the most common treatments for patients dealing with Bell’s palsy, partial facial paralysis or synkinesis. It helps reduce unwanted muscle movement in the normal side of a patient’s face. At the same time, Botox helps lower tension in the face that are hyperactive as a result of synkinesis.
Along with Botox, neuromuscular retraining may be used to help a patient improve facial symmetry and reduce the signs of facial paralysis. It must be performed by an expert physical therapist, who will teach a patient different facial muscle exercises that he or she can perform from any location, at any time.
In the senior author’s (BA) practice, neuromuscular rehabilitation and BTX-A are the primary treatment strategies for younger patients with limited facial palsy and minimal to moderate amount of synkinesis (Type B and C patients). In patients who are older and/or have a more dense paralysis, BTX-A and neuromuscular retraining are used to complement other surgical reanimation techniques. Facial fillers such as hyaluronic acids, calcium hydroxylapatite, and injectable poly-L-lactic acid are also used to improve facial volume asymmetry, which is commonly seen in patients with facial paralysis.
Surgical Intervention
Surgical intervention for facial paralysis can be divided into static and dynamic reanimation. Dynamic reanimation can be further subdivided into “volitional” and “spontaneous” reanimation. Volitional dynamic reanimation requires the patient to be conscious about moving the face, whereas spontaneous reanimation does not. Static surgical procedures only improve a patient’s facial symmetry at rest and technically do not reanimate the face.
To determine if a patient qualifies for surgery, a full assessment must be performed. A facial plastic and reconstructive surgeon evaluates a patient, as well as his or her symptoms and medical history. If a facial plastic and reconstructive surgeon believes a patient can benefit from surgery, a custom treatment plan is then developed. Or, if the risk of surgery is significant, this surgeon offers alternative treatment options to the patient.
Static Surgical Reconstruction
Static techniques are the workhorse of facial paralysis reconstruction. Static procedures are indicated for individuals who are not appropriate candidates for dynamic reanimation and/or regions of the face that are not amenable to dynamic reconstruction (e.g. brow ptosis, external nasal valve collapse). Static procedures include repair of brow ptosis, gold/platinum weight reconstruction for lagophthalmos, lower eyelid reconstruction, lower lip shortening, external nasal valve reconstruction, superficial musculoaponeurotic system (SMAS) rhytidectomy, and static sling suspension. (Figures 1A and 1B).
Figure 1A. Preoperative photo of patient with left-sided facial nerve paralysis.
Figure 1B. Postoperative photo of same patient after undergoing superficial
musculoaponeurotic system (SMAS) rhytidectomy and static sling suspension.
For lower facial paralysis reconstruction, static sling suspension is able to achieve two major goals: improve facial symmetry and reduce functional deficits such as oral incompetence, biting of inner gums and poor articulation. Since static slings do not interfere with nerve regeneration, it can be used to achieve an immediate aesthetic and functional improvement in conjunction with dynamic procedures such as cable nerve grafting, hypoglossal-facial nerve anastomosis and cross-facial nerve grafts. Static slings can also be used to augment muscle transfer techniques or revise previous dynamic reanimation procedures. As stand-alone procedures, static slings do not directly address the smile mechanism but do improve patient’s perception of their smile and face.
In our practice, static slings are offered to individuals who are not candidates for dynamic facial reanimation, such as those who have advanced age, partial paralysis with adequate oral commissure excursion, and major head and neck malignancy. In appropriate candidates, this procedure is considered only after the status of nerve regeneration has been ascertained; typically one year after date of onset or if the facial nerve has been deliberately sacrificed. Patients are typically elderly with a history of tumor ablation in the head and neck or cerebellopontine angle region.
Tensor fascia lata (TFL) is the ideal choice for static sling procedures.23 A substantial amount of tissue can be harvested from the lateral thigh and multiple strips can be created for both oral commissure suspension as well as external nasal valve repositioning. Although TFL does require a separate donor site that can increase patient morbidity, it has the advantage of being an autogenous material with long-term viability.
Commercially available, freeze-dried acellular human dermis (AlloDerm® Regenerative Tissue Matrix, LifeCell, Branchburg, NJ) has also been used for facial static slings. The advantage of acellular human dermis is that it precludes a donor site harvest. Acellular human dermis is readily available, integrates into surrounding tissue and can be customized quickly to create exact facial slings. Although there have been reports of poor long-term outcome with AlloDerm®, namely sling failure and infection, our experience has been satisfactory in both short and long-term use in a carefully selected patient population who would have significant morbidity from harvesting of TFL.24
Expanded polytetrafluoroethylene (ePTFE, Gore-tex®, Implantech Associates, Santa Barbara, CA) is a synthetic material that can also be considered for use in static sling procedures. Levet and Jost reported on their use of ePTFE for facial suspension in the French literature in 1987 with good results. 25 Petroff, Goode, and Levet also reported favorable results from facial suspensions with ePTFE soft-tissue patch without infection or extrusion. 26 Iwahira and Maruyama, in 1992, reported the use of ePTFE with temporalis transfer as “extensions” when the temporalis muscle fascia was weak and/or short. 27 ePTFE circumvents the need to harvest TFL thus eliminating a second donor site morbidity; however, given its lack of integration with local tissue, its use in our practice has been limited.
Suture suspension techniques that have been popularized in aesthetic midface lifts can also be successfully used for patients requiring minor repositioning of the oral commissure.27,28 Suture suspension techniques are generally less invasive than other static techniques and can be performed percutaneously and under local anesthesia; however, its potential for comprehensive suspension is limited and its long-term outcome has not yet been adequately studied.
The static sling procedure is typically performed simultaneously with a rhytidectomy. Using a rhytidectomy incision, a deep subcutaneous plane is elevated to the level of the oral commissure. ( Figure 2A.) A single sheet of 5 x 12 cm sling material (TFL, acellular dermis, ePFTE) is typically used to create an equal amount of tension along the oral commissure and nasolabial fold. ( Figure 2B.) The sling is sutured at the modiolus to orbicularis oris or subdermal tissue layer if orbicularis is significantly atrophied. Approximately three to five separate O-Vicryl sutures are used to attach the graft to the oral commissure and nasolabial fold. ( Figure 2C.) The sling should be slightly overcorrected in a posterolateral vector and secured to the deep temporalis fascia, zygomatic arch and preauricular SMAS. Additional sheet of sling material (1.5 x 10 cm) is typically used to elevate the nasal alar crease to improve external nasal valve collapse and nasal obstruction.
Dynamic Surgical Reconstruction
Although static reconstruction has significant advantages such as creating symmetry at rest and improving functional deficits, most patients’ primary goal of smiling remains unfulfilled. The goal of dynamic reanimation is to allow the patient to improve symmetry and regain a functional smile mechanism. As stated earlier, the smile mechanism may be volitional where the patient needs to be actively conscious of moving the face or spontaneous. Dynamic reanimation can be achieved using regional muscle transfers (temporalis), cranial nerve substitution techniques (hypoglossal-facial nerve transfer) and/or microvascular free muscle grafts.
Temporalis Transfer
The goal of temporalis muscle transfer is to attach a segment of the temporalis muscle to the oral commissure in order to recreate a smile mechanism by activating the motor branches of the trigeminal nerve. This is accomplished by biting down as if mimicking the chewing mechanism. Lexer, in 1908, was the first to describe regional muscle transfer utilizing the temporalis muscle. The procedure was modified in 1934 by Gillies who performed a temporal muscle flap by folding it over the zygoma to the level of the oral commissure subcutaneously.29 Since this technique led to fullness over the zygoma with hollowing in the temporal fossa, MacLaughlin in 1952 proposed temporalis muscle tendon transfer. In this technique, the temporalis muscle tendon was detached from the coronoid process and secured to the oral commissure using a fascial graft.30 In the 1990’s, Cheyney modified the classic temporalis flap by using only the middle third of the temporal muscle and obliterating the hollowness with a simultaneous temporoparietal fascial flap in an attempt to reduce the temple hollowness.31 In the 1990s, Labbé also improved temporalis contour irregularity by repositioning the posterior third of the temporalis muscle and inserting it into the lips after detaching it from the coronoid process and fracturing of the zygomatic arch.32 In 2007, Byrne reintroduced and modified the temporalis tendon transfer technique which has since gained significant acceptance.33
The advantages of the temporalis muscle or tendon transfer is that it can be performed as a single-stage dynamic reanimation procedure, does not interfere with native nerve regeneration, is relatively simple to perform and provides active excursion of their oral commissure. The temporalis muscle however does not provide spontaneous reanimation and the smile mechanism is often not utilized by patients due to lack of training. Overall, the role of temporalis muscle or tendon transfers have been limited in the senior author’s practice as other techniques such as gracilis free muscle grafts have supplanted this technique.
Hypoglossal-Facial Nerve Anastomosis
The first cranial nerve substitution procedure was attempted by Drobnik in 1879 where he attempted to anastomose the spinal accessory and facial nerves to rehabilitate patients with facial paralysis. 34 In 1895, Balance attempted an end-to-end neurorrhaphy without success. 35 Korte is credited with performing the first hypoglossal-facial anastomosis (XII-VII) in 1901 with long-term favorable outcomes.5 Since that time and with improvements in microsurgical technique, cranial nerve substitution techniques have been extensively utilized with satisfactory outcome.
The XII-VII transfer utilizes the hypoglossal nerve to neurotize a non-functional facial nerve (Type E patients with complete flaccid paralysis). The nerve input would increase muscle tone reversing the flaccidity of a complete paralysis. Tongue movement would then allow patients to control the facial musculature and consciously move the face. This approach is most appropriate for complete and permanent facial paralysis. This technique is commonly employed following surgery or trauma where the facial nerve is irreversibly injured and the proximal nerve stump is not accessible for primary or cable nerve repair (acoustic neuroma resection, temporal bone trauma, and skull base surgery).
Successful outcomes are seen only if the patient has an intact extracranial facial nerve (preferably the main trunk as it exists the stylomastoid foramen or the lower division), mimetic facial muscles, and donor hypoglossal nerve. Traumatic, iatrogenic or intentional injury to the facial nerve distal to the parotid gland precludes the use of this procedure. The major concerns with the XII-VII transfer is the potential of hemitongue paralysis leading to dysphagia and dysarthria.36 As a result, patients with multiple cranial neuropathies are not appropriate candidates. Other contraindications to this procedure include developmental facial paralysis, prolonged facial palsy of greater that two year duration and partial paralysis with existing muscle tone (Type A-D). Generally, patients who are unable to accept deficit of the hypoglossal nerve and/or have facial paralysis of more than two-year duration leading to irreversible facial muscle atrophy are poor candidates for XII-VII transfer.
Improved facial tone and symmetry occurs in over 90% of patients following hypoglossal-facial anastomosis and is usually seen within 4-6 months.37 Eyelid tone may improve the closure of the eye and thereby facilitate removal of gold weight. Voluntary facial movement begins after tone develops and continues to improve over the ensuing 18 months. Voluntary motion typically creates synkinetic mass facial movement of all muscles and is usually not targeted to excursion of the oral commissure. Younger patients and early nerve substitution have better overall outcomes.38,39
There are several hypoglossal-facial nerve techniques that could be utilized to achieve satisfactory outcome. The classic procedure involved the sacrifice of the entire hypoglossal nerve, which often leads to hemitongue paralysis and variable functional deficits such as dysphagia, dysarthria and hemitongue atrophy.40 The technique for this operation included performing a modified blair incision and exposing the hypoglossal nerve and facial nerve via a superficial parotidectomy. The hypoglossal nerve is then sacrificed and rotated up into the parotid gland and anastamosed to the facial nerve. This operation has been largely replaced by techniques that only partially sacrifice the hypoglossal nerve.
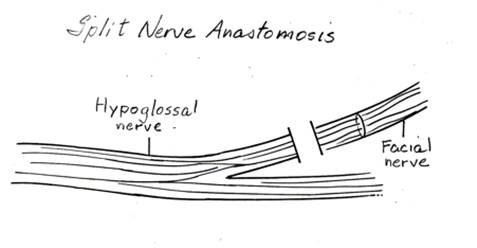
Several modifications have been described for the XII-VII transfer. The split XII-VII utilizes approximately 30-50% of the hypoglossal nerve width in a linear fashion,
Figure 3. Splitting of the hypoglossal nerve (CN XII) to be reanastomosed with the facial nerve.
which is then rotated up toward the parotid region and anastomosed to the lower division of the facial nerve.41 ( Figure 3.) By splitting the nerve, the risk of hemitongue atrophy is minimized, although long-term evaluation of the tongue may still reveal partial tongue atrophy. XII-VII jump graft, introduced by Terzis, avoids linear splitting of the hypoglossal nerve. The hypoglossal nerve is divided 25-50% in diameter and sewn to a cable nerve graft (great auricular or sural nerve graft). The nerve graft is then anastomosed to the main trunk of the facial nerve. Although this procedure requires harvesting of a nerve graft, the overall morbidity is lower than split XII-VII. Several authors have reported comparable functional results with lower incidence of hypertonia or mass facial movements.42,43
Facial nerve translocation is another useful XII-VII technique.44 The facial nerve in this procedure is identified in the vertical segment of the mastoid bone and translocated down to the hypoglossal nerve. The hypoglossal nerve is partially transected and primary anastomosis is performed. This technique circumvents the need to identify the facial nerve in the parotid gland and has the advantage of a single anastomosis. However, the time to recovery is lengthened since the facial nerve is anastomosed farther posteriorly in the mastoid.
The hypoglossal-facial nerve transfer is an extremely valuable tool in the armamentarium of a facial paralysis surgeon. Unfortunately, many patients who are excellent candidates are not seen in a timely fashion and miss the opportunity to have this procedure. The senior author often combines this procedure with static slings in order to obtain immediate results until the reinnervation is completed. ( Figure 4.)
In younger patients, we also combine this procedure with more advanced facial reanimation techniques such as cross facial nerve grafts (CFNG) and gracilis flaps which provide more spontaneous and targeted smile excursion. ( Figure 5.)
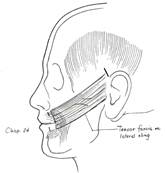 Figure 4. Placement of a static sling from the oral commissure to the temporal region. |
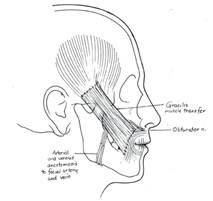
Figure 5. Placement of a racilis flap combined with cross facial nerve grafts to allow dynamic movement of the oral commissure. Such procedures allow patients more spontaneous ability to smile. |
Free Muscle Transfer
Although many of the techniques described in this chapter thus far provide excellent static reconstruction and dynamic reanimation, none truly results in spontaneous facial reanimation. Direct facial nerve repair as described in chapter XX and free muscle transfer powered by a CFNG are the only surgical procedures that can restore spontaneous reanimation of the face. Since direct nerve repair can often cause significant synkinesis, CFNG with free muscle transfer is the only procedure that can actually provide a targeted smile mechanism.
Initially described by Scaramella in 1970, the CFNG was subsequently modified by Smith, Anderl, Fisch, and Conley.45-48 ( Figure 6.)
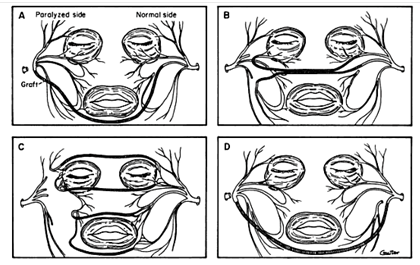
Reprinted with permission from The Facial Nerve: May’s Second Edition, May/Schaitkin, 2009, Thieme.
Scaramella initially used the CFNG as a neural input to the dysfunctional facial nerve. He utilized a sural nerve graft to connect the cervical branch of the normal facial nerve and the abnormal facial nerve trunk. The general results with this approach were less than ideal and these techniques were mostly abandoned. 49-51
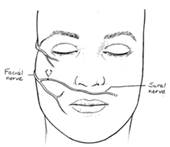
Figure 7. Two stage procedure of CFNG and free muscle graft. Figure 7A (left). Utliization of the sural nerve as a CFNG to extend the nerve across the midline of the face. Figure 7B (right). Combination of the CFNG with a free muscle graft (such as a gracilis free muscle).
It wasn’t until the CFNG and free muscle grafts were combined in a two-stage operation that this surgical approach proved to be successful in restoring spontaneous facial movement. In 1979, Harii recommended the two-stage procedure utilizing both the CFNG as neural source and gracilis free muscle transfer as muscle source. ( Figure 7.) Although the two stage cross facial nerve graft technique proved to be reproducible, patients were not satisfied with the muscle bulk. In 1984, Manktelow addressed this problem by using a ‘mini gracilis’ transfer, which is now the accepted manner for this technique.52-58
The gracilis muscle has also been utilized in other manners for facial reanimation. Introduced by O’Brien and popularized by Kumar in the 1990’s, the one-stage gracilis muscle transfer allowed reduction of the rehabilitation period by 10 months. The technique places the neurovascular pedicle at the nasolabial fold and transfers the obturator nerve through the upper lip to the contralateral facial nerve.55 Although the two-stage gracilis procedure resulted in better symmetry at rest (67% vs 20% for the single-stage), the one-stage gracilis transfer otherwise produced comparable results with 90% of single-stage patients and 93% of two-stage patients having good results.56
Zuker and Manktelow also popularized the use of gracilis muscle as a single stage procedure by using the masseteric branch of the trigeminal nerve as the neural source.52 ( Figure 8.) This approach was first utilized in patients with moebius syndrome who have bilateral facial paralysis but later expanded to unilateral facial palsy. Although patients obtain excellent excursion of the oral commissure, this approach is limited by its lack of spontaneity. The trigeminal-gracilis flap thus appears to be an excellent alternative to temporalis muscle or tendon transfer as a single-stage dynamic facial reanimation procedure.
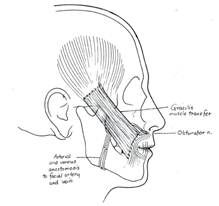
Figure 8. Gracilis muscle transfer with anastomosis of the obturator nerve to the CFNG and the adductor artery and vein to the facial artery and vein, respectively. Latissimus dorsi and pectoralis minor free muscle transfers have also been utilized in dynamic reanimation.57-63 Although the latissimus dorsi muscle could be used as single stage operation due to the appropriate length of the thoracodorsal nerve, it usually creates excessive bulk. The pectoralis minor muscle introduced by Terzis and Manktelow60 and subsequently popularized by Harrison is an excellent free muscle source that provides similar outcomes to gracilis and can be performed with a limited amount of muscle.
Currently, the senior author’s preferred treatment option for patients under the age of 55 with unilateral complete facial nerve paralysis is the two-stage CFNG followed by gracilis free muscle transfer. We also use this option for selected patients with Type D facial palsy who have severe synkinesis and a “frozen smile”.
In the CFNG procedure, a large branch of the peripheral facial nerve on the unaffected side is identified just past the parotid gland via a deep-plane facelift approach. (Figure 9.)
Figure 9. Proposed facelift incision and identification of the peripheral facial nerve on the unaffected side.
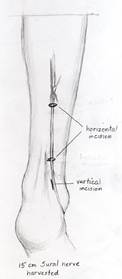
A nerve stimulator is utilized to ensure that the nerve only stimulates the zygomaticus muscles and does not innervate the oribicularis oculi. A 15 cm segment of sural nerve is harvested and primary neurorrhaphy is performed between the sural and peripheral facial nerve. ( Figure 10A.) The distal segment of the sural nerve is tunneled to the contralateral gingivobuccal sulcus and tagged with a hemoclip and prolene suture. (Figure 10B.)
 |
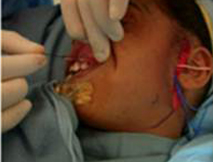 |
| Figure 10A. Harvest of approximately 15cm of the sural nerve, which is located approximately 1cm posterior to the lateral malleolus. | Figure 10B. The distal segment of the sural nerve is tunneled to the contralateral gingivobuccal sulcus and tagged with a hemoclip and prolene suture. |
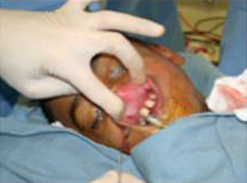
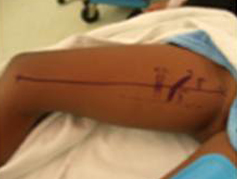
The gracilis free flap muscle transfer is performed approximately 6–12 months later once a positive Tinel’s sign is identified signifying axonal growth through the cross facial nerve graft. During the second stage, the sural nerve graft is first biopsied in the gingivobuccal sulcus to confirm nerve growth. A segment of gracilis muscle is harvested (20-30 gm) with the obturator nerve and adductor artery/vein (Figure 11).
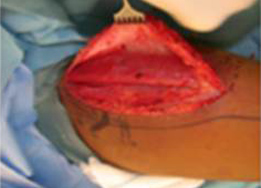
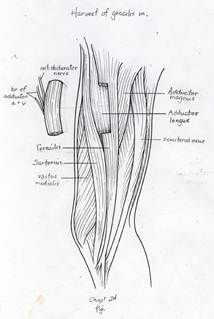
A deep subcutaneous flap is then elevated on the paralytic side via a modified blair incision and sub-SMAS plane is entered at the anterior border of the parotid gland. The facial artery and vein are identified at the mandibular border and prepared for revascularization of the gracilis muscle. The sub-SMAS plane is continued to the oral commissure and nasolabial fold. Figure 12.
 Figure 12A (left). Marking of the modified blair incision. |
 Figure 12B (right) Continuation of the sub-SMAS plane to the oral commissure and nasolabial fold. |
Four to five O-vicryl sutures are placed at the modiolus and nasolabial folds. The gracilis muscle is then secured to these sutures and parachuted into the oral commissure (Figure 13).
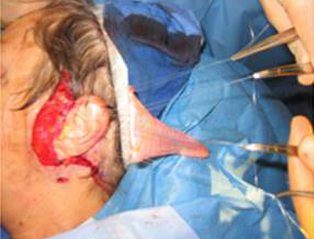
Arterial and venous anastomosis is completed under operating microscope and the obturator nerve is tunneled to the gingivobuccal sulcus where neurorrhaphy is performed with the cross facial sural nerve graft.
Gracilis movement typically begins 6 months after the operation and continues to strengthen over the ensuing year. The most common adverse event with this procedure includes lateralization of the nasolabial fold, excessive flap bulk and infection. About 10-20% of patients will require a third stage procedure for reconfiguration of the nasolabial fold and/or debulking of the muscle.
Lower Lip Reconstruction
Marginal mandibular nerve paresis leads to lower lip immobility as a result of depressor labii inferioris dysfunction. This lack of mobility is most pronounced during speech when the paralyzed side is elevated while the non-paralyzed side moves inferiorly. The senior author’s preference is to manage this problem with chemodenervation (BTX-A) of the non-paralyzed depressor labii inferioris.64 Chemodenervation of the normal side reduces the visibility of the dentition as well as the mobility of the lower lip on the non-paralyzed side during speech and animation. This simple procedure can have a dramatic impact in the overall appearance of patients, especially those with full denture smile pattern as well as individuals who suffer from congenital unilateral lower lip palsy (CULLP). 65
Other approaches include: selective myectomy of the innervated depressor labii inferioris, sectioning the contralateral marginal branch, anterior belly of digastric transfer, free extensor digitorum brevis transfer and platysma transfer.66-71 Although these options have been extensively discussed in the literature, they are rarely useful in clinical practice. In our experience, non-invasive injection of BTX-A to the innervated lower lip depressors is a simple and reproducible option with extremely high patient satisfaction and acceptance.
Bilateral Paralysis
Bilateral facial paralysis, most commonly associated with Moebius syndrome, remains as one of the most challenging areas in facial reanimation. While symmetry exists, patients cannot express any emotions and have significant functional and psychosocial deficits. Historically, bilateral temporalis muscle transfer was the treatment of choice.35 However, as free muscle transfer techniques have evolved, they have gradually replaced local muscle transfers with significantly better outcome. 72 Potential motor donor nerves include the masseteric branch of the trigeminal nerve, partial hypoglossal nerve, accessory nerve, and cervical motor donors from the cervical plexus and C7 nerve root.73 Popularized by Zuker and Manktelow, gracilis free muscle flap innervated by the masseteric branch of the trigeminal nerve is the treatment of choice for this patient population.72 This approach does not produce spontaneous smile mechanism but with aggressive neuromuscular rehabilitation, most patients obtain satisfactory outcome. Bilateral procedures can be performed either in a single or two-stage manner preferably before the age of 7.22
Conclusion
The treatment of long-standing facial paralysis with the ultimate goal of symmetry and spontaneous animation has challenged reconstructive surgeons for decades. The evolution of static and dynamic surgical techniques has vastly improved our ability to deliver better outcome. Surgical and non-surgical options must be employed in order to obtain the best possible outcomes.
References
1. Bell C. “On the Nerves: Giving an account of some experiments on their structure and function which leads to a new arragement of the systems.” Phil Trans Roy Soc (Biol) 3:398, 424, 1821.
2. Van de Graaf RC, Nicolai JP. “Bell’s palsy before Bell: Cornelis Stalpart van der Wiel’s observation of Bell’s palsy in 1683.” Otol Neurotol 26. 2005. pp. 1235-1238.
3. Drobnick, cited by Sawicki B, in Chepault: The Status of Neurosurgery. Paris. J. Reuff, 1902. P. 189.
4. Manasse P: Uber Vereinigung des N. facialis mit dem N. accesorius durch die Nervenpfropfung (Greffe nerveuse). Arch Klin Chir. 62: 805, 1900.
5. Korte W. Ein Fall von Nervenpfropfung: des Nervus facialis auf den Nervus hypoglossus. Deutsche med Wihnschr 1903; 17: 293-295
6. Stacke L., quoted by Alt. F: The operative treatment of otogenic facial palsy.” Verhundl Deutsch Otol Geselisch 17:190, 1908.
7. Bunnell S: Suture of the facial nerve within the temporal bone with a report of the first successful case. Surg Gynecol Obstet 45:7. 1927.
8. Lathrop FD: Facial nerve surgery in the European theather of operation. Laryngoscope 54:665-676. 1946.
9. Myers D: war injuries to the mastoid and facial nerve. Arch Otolaryngol 44:392-405. 1946.]
10. Van de Graaf RC, Nicolai. JP “Was Thomasz Drobnik really the first to operate on the facial nerve?” Otol Neurotol 24. 2003. pp. 686–690.
11. Miehike A. Surgery of the Facial Nerve (2nd ed.), WB Saunders, Philadelphia (1973).
12. Lexer E, Eden R: Uber die chirurgische Behandlung der peripheren Facialislahmung. Beitr Klin Chir 73:116,1911.
13. Erlacher P. Direct and muscular neurotization of paralyzed muscle. Am J Ortho Surg. 13:22-32. 1915.
14. Coulson SE, O’Dwyer NJ, et al. “Expression of emotion and quality of life after facial nerve paralysis.” Otol Neurotol 25 (2004), pp. 1014–1019.
15. Scaramella LF. “Preliminary report on facial nerve anasotomosis. Second international symposium on Facial Nerve Surgery. Osaka, Japan 1970.
16. Anderl H “Reconstruction of the face through cross-face nerve transplantation in facial paralysis. Chir Plast. 2:17.1973.
17. Owen N: Surgical correction of facial paralysis. Plast Reconstr Surg 2:25. 1947.]
18. Thompson N. Autogenous free grafts of skeletal muscles. Plast Reconstr Surg. 48:11.1971.
19. Ruben L: Reanimation of the Paralyzed Face. St Louis, C.V. Mosby Co., 1977.]
20. Harii K, Ohmori K, Torii S. “Free gracilis muscle transplantation with neurovascular anastomosis for the treatment of facial paralysis.” Plast Reconstr Surg. 57: 133-143. 1976.
21. McCabe BF. “Facial Nerve Grafting.” Plastic & Reconstructive Surgery: January 1970 – Volume 45 – Issue 1 – ppg 70-75
22. Laskawi R. “Combination of Hypoglossal-Facial Nerve Anastomosis and Botulinum-Toxin Injections to Optimize Mimic Rehabilitation after Removal of Acoustic Neurinomas.” Plas & Recon Surg. 99(4). April 1997. P. 1006-1011.
23. McLaughlin CR. “Surgical Support in Permanent Facial Paralysis.” Plastic & Recon Surg. 11(4). P. 302-314. 1953.
24. Constantinides M, Doud Galli SK, Miller PJ. “Complications of Static Facial Suspensions With Expanded Polytetrafluoroethylene (ePTFE).”The Laryngoscope. 111(12). Dec 2001.p 2114–2121.
25. Levet Y, Jost G. Utilisation du polytetrafluoroethylene (Gore-Tex e-PTFE Soft Tissue Patch) dans les suspensions de paralysies faciales anciennes et en tissu de comblement. Ann Otolaryngol Chir Cervicofac 1987; 104: 65–69.
26. Petroff MA, Goode RL, Levet Y. Gore-Tex implants: applications in facial paralysis rehabilitation and soft-tissue augmentation. Laryngoscope 1992; 102: 1185–1189.
27. Iwahira Y, Maruyama Y. The use of Gore-Tex soft tissue patch to assist temporal muscle transfer in the treatment of facial nerve palsy. Ann Plast Surg 1992; 29: 274–277.
28. Heffelfinger RN, Blackwell KE, Rawnsley J, Keller GS. “A Simplified Approach to Midface Aging.” Arch Facial Plast Surg. 2007;9(1):48-55.
29. Gilles H “Experience with fascia lata grafts in the operative treatment of facial paralysis.” Proc R Soc Med 27 (1934) p.1372.
30. Cheney ML, McKenna MJ, et. al. “Early Temporalis Muscle Transposition for the Management of Facial Paralysis.” Laryngoscope. 105. September 1995. P. 993-1000.
31. Labbe D. “Lengthening of temporalis myoplasty and reanimation of lips. Technical notes.” Ann Chir Plast Esthet 42 (1997), pp. 44–47.
32. Byrne PJ, Kim M, et. al. “Temporalis Tendon Transfer as Part of a Comprehensive Approach to Facial Reanimation.” Arch Facial Plast Surg. 2007;9(4):234-241.
33. Byrne PJ, Kim M, Boahene K, Millar J, Moe K. “Temporalis Tendon Transfer as Part of a Comprehensive Approach to Facial Reanimation” Arch Facial Plast Surg. 2007;9(4):234-241.
34. Sawicki B, Drobnik T, de Posen IN. Chipault A L’etat Actual de la Chirurgie Nerveuse. Paris; J. Rueff 1903.
35. Balance CA, Gallance HA, Stewart P. Remark on the operative treatment of chronic facial palsy of the pripheral origin. Br J Med 1903; May 2: 1009-1015.
36. May M, Schaitkin B. The Facial Nerve. Second Ed. Thieme. New York, NY. 1996 623-4.
37. Baker D. “Hypoglossal facial nerve anastomosis indications and limitations.” Proceedings Fifth International Symposium Facial Nerve. New York. Masson Publ. 1985. Pp 526-529.
38. Gavron GP, Clemis JD. “Hypoglossal-Facial Nerve Anastomosis: A Review of Forty Cases Caused by Facial Nerve Injuries in the Posterior Fossa.” Laryngoscope 94: 1447-1450.
39. Conley J. “Hypoglossal-Facial Anastomosis.” Neurological Surgery of the Ear and Skull Base. New. York. Raven Press. 1983. 93-98.
40. Pensak ML. Controversies In Otolaryngology. Thieme. New York, NY. 2001. P.130.
41. Conley J. Baker D. “Hypoglossal–facial nerve anastomosis for innervation of the paralyzed face.” Plast Reconstr Surg. 63:3-72. 1979.
42. Snow JB. Ballenger’s Manual of Otorhinolaryngology – Head and Neck Surgery. BC Decker Inc. Hamilton, Ontario. 2003. P.215
43. Scaramella LF. “Cross-Face Facial Nerve Anastomosis: Historical Notes.” Ear Nose Throat J. 76:343-354. 1996.
44. Slattery III WH, Wilkinson EP. “Facial translocation for hypoglossal-facial anastomosis.” Oral presentation, XI International Facial Nerve Symposium. (Rome, Italy, April 25-28, 2009.)
45. Smith JW. “A New Technique of Facial Animation. Transactions of the Fifth International Congress of Plastic Surgery.” Butterworths, Australia. 1971. pp. 83.
46. Anderl H. “Cross-Face Nerve Grafting – Up to 12 Months of Seventh Nerve Disruption.” Reanimation of the Paralyzed Face. St. Louis, Mosby. 1977. Pp. 241-77.
47. Fisch U. “Facial Nerve Grafting.” Otol Clin North Am. 7:517-524. 1974.
48. Kunihior T, Kanzaki J, et. al. “Hypoglossal-Facial Nerve Anastomosis After Acoustic Neuroma Resection: Influence of the Time of Anastomosis on Recovery of Faacial Movement.” J Oto Rhino Laryngol. 58:32-35. 1996.
49. Stennert EJ. “Hypoglossal Facial Anastomosis: Its Significance for Modern Facial Surgery.” Clin Plast Surg. 6:471-486. 1979.
50. Holtege G, Kuypers HG, et. al. “The Organization of the Bulbar Fiber Connections to the Trigeminal, Facial, and Hypoglossal Motor Nuclei II. An autoradiographic tracing study in the cat.” Brain. 100:264-286. 1977.
51. Harii K. “Microneurovascular free muscle transplantation for reanimation of facial paralysis.” Clin Plast Surg 6 (1979), pp. 361–375.
52. Manktelow RT. “Free muscle transplantation for facial paralysis.” Clin Plast Surg 11 (1984), pp. 215–220.
53. O’Brien BM, Pederson WC, et. al. “Results of management of facial palsy with microvascular free-muscle transfer.” Plast Reconstr Surg 86 (1990), pp. 12–22 discussion 23–24.
54. Kumar PA. “Cross-face reanimation of the paralysed face, with a single stage microneurovascular gracilis transfer without nerve graft: a preliminary report.” Br J Plast Surg 48 (1995), pp. 83–88.
55. Kumar PA and Hassan KM. “Cross-face nerve graft with free-muscle transfer for reanimation of the paralyzed face: a comparative study of the single-stage and two-stage procedures.” Plast Reconstr Surg 109 (2002), pp. 451–462 discussion 63–64.
56. Chuang DC. “Technique evolution for facial paralysis reconstruction using functioning free muscle transplantation–experience of Chang Gung Memorial Hospital.” Clin Plast Surg 29 (2002), pp. 449–459
57. Dellon AL and Mackinnon SE. “Segmentally innervated latissimus dorsi muscle. Microsurgical transfer for facial reanimation.” J Reconstr Microsurg 2 (1985), pp. 7–12.
58. Mackinnon SE and Dellon AL. “Technical considerations of the latissimus dorsi muscle flap: a segmentally innervated muscle transfer for facial reanimation.” Microsurgery 9 (1988), pp. 36–45.
59. Hata Y, Yano K, et. al. “Treatment of chronic facial palsy by transplantation of the neurovascularized free rectus abdominis muscle.” Plast Reconstr Surg 86 (1990), pp. 1178–1187 discussion 88–89.
60. Terzis JK and Manktelow RT. “Pectoralis Minor: a new concept in facial reanimation.” Plast Surg Forum 5 (1982), pp. 106–110.
61. Harrison DH. “The pectoralis minor vascularized muscle graft for the treatment of unilateral facial palsy.” Plast Reconstr Surg 75 (1985), pp. 206–216.
62. Terzis JK. “Pectoralis minor: a unique muscle for correction of facial palsy.” Plast Reconstr Surg 83 (1989), pp. 767–776.
63. Tulley P, Webb A, et. al. “Paralysis of the marginal mandibular branch of the facial nerve: treatment options.” Br J Plast Surg 53 (2000), pp. 378–385.
64. Curtin JW, Greeley PW, et. al. “A supplementary procedure for the improvement of facial nerve paralysis.” Plast Reconstr Surg Transplant Bull 26 (1960), pp. 73–79.
65. Lindsay RW, Edwards C, Smitson C, Cheney ML, Hadlock TA. “A systematic algorithm for the management of lower lip asymmetry. Vol 32(1). P. 1-7. 2011.
66. Niklison J. “Contribution to the subject of facial paralysis.” Plast Reconstr Surg (1946) 17 (1956), pp. 276–293.
67. Edgerton MT. “Surgical correction of facial paralysis: a plea for better reconstructions.” Ann Surg 165 (1967), pp. 985–998.
68. Conley J, Baker DC, et. al. “Paralysis of the mandibular branch of the facial nerve.” Plast Reconstr Surg 70 (1982), pp. 569–577.
69. Terzis JK and Kalantarian B. “Microsurgical strategies in 74 patients for restoration of dynamic depressor muscle mechanism: a neglected target in facial reanimation.” Plast Reconstr Surg 105 (2000), pp. 1917–1931 discussion 32–34.
70. Mayou BJ, Watson JS, et. al. “Free microvascular and microneural transfer of the extensor digitorum brevis muscle for the treatment of unilateral facial palsy.” Br J Plast Surg 34 (1981), pp. 362–367.
71. Terzis JK and Konofaos P. “Nerve transfers in facial palsy.” Facial Plast Surg 24 (2008), pp. 177–193.
72. Harrison DH. “The treatment of unilateral and bilateral facial palsy using free muscle transfers.” Clin Plast Surg 29 (2002), pp. 539–549 vi.
73. Zuker RM, Goldberg CS, et. al. “Facial animation in children with Moebius syndrome after segmental gracilis muscle transplant.” Plast Reconstr Surg 106 (2000), pp. 1–8
Request your consultation with Dr. Azizzadeh today
Call us at (310) 657-2203 to schedule an appointment.
Schedule a Consultation